|
|
||||||||||||||||||||||||||||||||||||||
|
HOME | BIOLOGY | FILMS | GEOGRAPHY | HISTORY | INDEX | INVESTORS | MUSIC | NEWS | SOLAR BOATS | SPORT |
||||||||||||||||||||||||||||||||||||||
|
A truly autonomous ship needs to be independent in terms of energy. By that I mean that for true endurance autonomy a ship needs to be able to survive on energy that it derives from renewable sources: nature. On the assumption that we have our sums right, and that energy can be harnessed to keep our robot moving at a respectable pace, then we need to store some of the energy for the few times that nature is taking a rest. For that we need batteries, and we need batteries that are light and do not suffer from memory effect and other nasty side effects that nickel cadmium and to a lesser extent nickel metal hydride. For our project this leaves lithium batteries - of which there are many types.
A lithium-ion battery (sometimes Li-ion battery or LIB) is a family of rechargeable battery types in which lithium ions move from the negative electrode to the positive electrode during discharge, and back when charging. Chemistry, performance, cost, and safety characteristics vary across LIB types. Unlike lithium primary batteries (which are disposable), lithium-ion electrochemical cells use an intercalated lithium compound as the electrode material instead of metallic lithium.
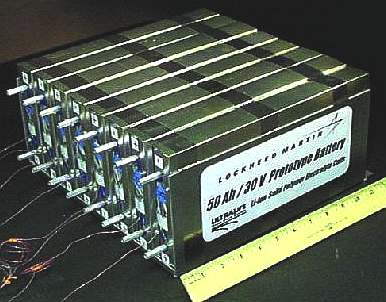
Lockheed lithium ion battery for NASA
During discharge, lithium ions Li+ carry the current from the negative to the positive electrode, through the non-aqueous electrolyte and separator
diaphragm.
Cylindrical 18650 lithium iron phosphate cell before closing
The three primary functional components of a lithium-ion battery are the anode, cathode, and electrolyte. The anode of a conventional lithium-ion cell is made from
carbon, the cathode is a metal oxide, and the electrolyte is a lithium salt in an organic
solvent.
Li-ion cells are available in various formats, which can generally be divided into four
groups:
The lack of case gives pouch cells the highest
energy density; however, pouch cells (and prismatic cells) require an external means of containment to prevent expansion when their state-of-charge (SOC) level is
high.
Varta lithium-ion battery, Museum Autovision, Altlussheim, Germany
Lithium metal is not directly compatible with water. However, the high gravimetric capacity of lithium metal, 3800 mA/g, and its highly negative standard electrode potential, Eo = -3.045 V, make it extremely attractive when combined as an electrochemical couple with oxygen or water. At a nominal potential of about 3 volts, the theoretical specific energy for a lithium/air battery is over 5000 Wh/kg for the reaction forming LiOH (Li + ¼ O2 + ½ H2O = LiOH) and 11,000 Wh/kg for the reaction forming Li2O2 (Li + O2 = Li2O2) or for the reaction of lithium with seawater, rivaling the energy density for hydrocarbon fuel cells and far exceeding Li-ion battery chemistry that has a theoretical specific energy of about 400 Wh/kg. With the invention of the protected lithium electrode (PLE), PolyPlus has introduced a unique technology that makes lithium metal electrodes compatible with aqueous and aggressive non-aqueous electrolytes, and enables the development of a new class of high energy density batteries.
PolyPlus uses a solid electrolyte membrane to prevent direct electron transfer from the negative electrode to species in the aqueous electrolyte, therefore extending the voltage window from the oxidative limit of the aqueous electrolyte to the lithium electrode potential (~ 4.5 V). This technology allows the construction of practical aqueous lithium batteries with cell voltages similar to those of conventional Li-ion or lithium primary batteries, but with much higher energy density (for H2O or O2 cathodes). We have observed that the PLE is remarkably stable to aqueous electrolytes and does not appear to be susceptible to parasitic side reactions that can lead to self-discharge in batteries. The availability of a PLE enables the development of a new class of stable, high voltage (~ 3 V) aqueous batteries with exceptionally high energy density (> 1000 Wh/l & Wh/kg).
HISTORY
Lithium batteries were first proposed by M.S. Whittingham, now at Binghamton University, while working for Exxon in the
1970s. Whittingham used titanium(II) sulfide as the cathode and lithium metal as the anode.
Nissan Leaf's lithium-ion battery pack
In 1991,
Sony and Asahi Kasei released the first commercial lithium-ion battery.
The three participants in the electrochemical reactions in a lithium-ion battery are the anode, cathode, and electrolyte.
Nokia Li-ion battery for powering a mobile phone
Electrode material Average potential difference Specific capacity Specific energy
Note that both advantages and disadvantages depend on the materials and design that make up the
battery. This summary reflects older designs that use carbon anode, metal oxide cathodes, and lithium salt in an organic solvent for the electrolyte.
Optima ACP TZERO tubular plate lead acid batteries
Wide variety of shapes and sizes efficiently fitting the devices they power.
Much lighter than other energy-equivalent secondary batteries.
High open circuit voltage in comparison to aqueous batteries (such as lead acid, nickel-metal hydride and nickel-cadmium). This is beneficial because it increases the amount of power that can be transferred at a lower current.
Self-discharge rate of approximately 5-10% per month, compared to over 30% per month in common nickel metal hydride batteries, approximately 1.25% per month for Low Self-Discharge NiMH batteries and 10% per month in nickel-cadmium batteries. According to one manufacturer, lithium-ion cells (and, accordingly, "dumb" lithium-ion batteries) do not have any self-discharge in the usual meaning of this word. What looks like a self-discharge in these batteries is a permanent loss of capacity (see Disadvantages). On the other hand, "smart" lithium-ion batteries do self-discharge, due to the drain of the built-in voltage monitoring circuit.
Components are environmentally safe as there is no free lithium metal.
Charging forms deposits inside the electrolyte that inhibit ion transport. Over time, the cell's capacity diminishes. The increase in internal resistance reduces the cell's ability to deliver current. This problem is more pronounced in high-current applications. The decrease means that older batteries do not charge as much as new ones (charging time required decreases proportionally).
The internal resistance of standard (Cobalt) lithium-ion batteries is high compared to both other rechargeable chemistries such as nickel-metal hydride and nickel-cadmium, and LiFePO4 and lithium-polymer cells. Internal resistance increases with both cycling and age. Rising internal resistance causes the voltage at the terminals to drop under load, which reduces the maximum current draw. Eventually increasing resistance means that the battery can no longer operate for an adequate period.
If overheated or overcharged, Li-ion batteries may suffer thermal runaway and cell rupture.[56] In extreme cases this can lead to combustion. Deep discharge may short-circuit the cell, in which case recharging would be
unsafe. To reduce these risks, Lithium-ion battery packs contain fail-safe circuitry that shuts down the battery when its voltage is outside the safe range of 3–4.2 V per
cell. When stored for long periods the small current draw of the protection circuitry itself may drain the battery below its shut down voltage; normal chargers are then ineffective. Many types of lithium-ion cell cannot be charged safely below 0°C. These devices occupy useful space inside the cells, add additional points of failure and irreversibly disable the cell when activated. They are required because the anode produces heat during use, while the cathode may produce oxygen. These devices and improved electrode designs reduce/eliminate the risk of fire or explosion.
Specific energy density: 150 to 250 W·h/kg (540 to 900
kJ/kg) Because lithium-ion batteries can have a variety of cathode and anode materials, the energy density and voltage vary accordingly.
The charging procedures for single Li-ion cells, and complete Li-ion batteries, are slightly different.
A Li-ion battery (a set of Li-ion cells in series) is charged in 3 stages:
2.
Balance (not required once a battery is balanced)
The increasing demand for batteries has led vendors and
academics to focus on improving the power density, operating temperature, safety, durability, charging time, output power, and cost of LIB solutions.
A lithium-ion battery from a laptop computer
Avoid deep discharge and instead charge more often between uses, the smaller the depth of discharge, the longer the battery will last.
Li-ion batteries require a battery management system to prevent operation outside each cell's safe operating area (over-charge, under-charge, safe temperature range) and to balance cells to eliminate SOC mismatches, significantly improving battery efficiency and increasing overall
capacity. As the number of cells and load currents increase, the potential for mismatch also
increases. There are two kinds of mismatch in the pack: state-of-charge (SOC) and capacity/energy ("C/E") mismatch. Though SOC is more common, each problem limits pack capacity (mA·h) to the capacity of the weakest cell.
Lithium-ion batteries can rupture, ignite, or explode when exposed to high temperature. Short-circuiting a battery will cause the cell to overheat and possibly to catch fire. Adjacent cells may then overheat and fail, possibly causing the entire battery to ignite or rupture. In the event of a fire, the device may emit dense irritating
smoke.
In March 2007, Lenovo recalled approximately 205,000 batteries at risk of explosion. In August 2007, Nokia recalled over 46 million batteries at risk of overheating and
exploding. One such incident occurred in the Philippines involving a
Nokia N91, which uses the BL-5C
battery.
In January 2008, the
United States Department of Transportation ruled that passengers on commercial aircraft could carry lithium batteries in their checked baggage if the batteries are installed in a device. Types of batteries affected by this rule are those containing lithium, including Li-ion, lithium polymer, and lithium cobalt oxide chemistries. Lithium-ion batteries containing more than 25 grams (0.88 oz) equivalent lithium content (ELC) are exempt from the rule and are forbidden in
air travel. This restriction greatly reduces the chances of the batteries short-circuiting and causing a
fire.
Researchers are working to improve the power density, safety, recharge cycle, cost and other characteristics of these batteries.
LINKS:
"Rechargeable Li-Ion OEM Battery Products". Panasonic.com. "Panasonic Develops Higher-Capacity Li-Ion Cells; Application of Silicon-based Alloy in Anode". greencarcongress.com The effect of PHEV and HEV duty cycles on battery and battery pack performance (PDF). 2007 Vapor-grown carbon fiber anode for cylindrical lithium batteries. Journal of Power Sources Battery Types and Characteristics for HEV ThermoAnalytics, Inc., 2007 Ballon, Massie Santos (14 October 2008). "Electrovaya, Tata Motors to make electric Indica". cleantech.com. Cleantech Group Thackeray, Thomas, and Whittingham (March 2000). Mixed Conductors for Lithium Batteries. mrs.com; Materials Research Society MSDS: National Power Corp Lithium Ion Batteries (PDF). tek.com; Tektronix Inc., 7 May 2004 Battery Management Systems for Large Lithium-Ion Battery Packs page 2 "Cell boards for various cell formats". Elithion.com Battery Management Systems for Large Lithium-Ion Battery Packs page 234 "USPTO search for inventions by "Goodenough, John"". Patft.uspto.gov US 4304825, Basu; Samar, "Rechargeable battery", issued 8 December 1981, assigned to Bell Telephone Laboratories Gholamabbas Nazri, Gianfranco Pistoia (2004). Lithium batteries: science and ... - Google Books. Springer Voelcker, John (September 2007). Lithium Batteries Take to the Road IEEE Spectrum. US 4668595, Yoshino; Akira, "Secondary Battery", issued 10 May 1985, assigned to Asahi Kasei Padhi, A. K. (1997). "Phospho-olivines as positive-electrode materials for rechargeable lithium batteries". Electrochem. Society Editors (6 March 2008). "In search of the perfect battery" (PDF). The Economist Staff (November 2003) (PDF). Lithium Ion technical handbook. Gold Peak Industries Ltd. "Impedance Analysis of Silicon Nanowire Lithium Ion Battery Anodes" (PDF) C. K. Chan; X. F. Zhang, Y. Cui (2007). "High Capacity Li-ion Battery Anodes Using Ge Nanowires" (PDF) Liquid Electrolyte Systems for Advanced Lithium Batteries (PDF). cheric.org; Chemical Engineering Research Information Center "A123 M1 cell specifications". Battery Management Systems for Large Lithium-Ion Battery Packs page 12 Buchmann, Isidor (200804). "Choosing a battery that will last". Isidor Buchmann (CEO of Cadex Electronics Inc.) Battery Management Systems for Large Lithium-Ion Battery Packs page 229 Buchmann, Isidor (September 2006). "BatteryUniversity.com: How to prolong lithium-based batteries" Buchmann, Isidor (February 2003). "Advanced battery analyzers". Isidor Buchmann "Lithium-ion Battery Charging Basics". PowerStream Technologies AeroVironment achieves electric vehicle fast-charge milestone avinc.com; AeroVironment, 30 May 2007 "Charging Lithium-ion Batteries". batteryuniversity.com. "The 3 charging stages". Liionbms.com. http://liionbms.com/php/wp_charging_stages.php Battery Management Systems for Large Lithium Ion Battery Packs section 6.2.3 Kevin Jost [ed.] (October 2006). Tech Briefs: CPI takes new direction on Li-ion batteries (PDF) Voelcker, John (September 2007). Lithium Batteries Take to the Road. IEEE Spectrum. Loveday, Eric (23 April 2010). "Hitachi develops manganese cathode, could double life of li-ion batteries". Nikkei (29 November 2009). Nissan On Track Nickel Manganese Cobalt Li-ion Cell for 2015 Green Car Congress Bulkeley, William M. (26 November 2005). "New Type of Battery Offers Voltage Aplenty, at Premium" (2 November 2005) A123Systems Launches Higher-Power, Faster Recharging Green Car Congress. "Imara Corporation website". Imaracorp.com. http://www.imaracorp.com. Retrieved 8 October 2011. Battery Company Says Its Technology Boosts Hybrid Battery Performance Green Car Advisor; Edmunds Inc. "A multifunctional 3.5 V iron-based phosphate cathode for rechargeable batteries" "A Research First: Lithium Air Battery Development (Press Release)". 17 November 2009 "Vanadium Modified LiFePO4 Cathode for Li-ion Batteries" Acceptance of the First Grid-Scale, Battery Energy Storage System" (Press release) 21 November 2008 Marty Ozols (11 November 2009). Altair Nanotechnologies Power Partner - The Military "Microsoft PowerPoint - 061125 Altair EDTA Presentation". Altairnano.com. Blain, Loz (2 November 2007). "Subaru doubles the battery range on its electric car concept". gizmag. "Li-Ion Rechargeable Batteries Made Safer". Nikkei Electronics Asia. 29 January 2008. "gatech.edu". http://www.mse.gatech.edu. Palca, Joe (6 April 2009). Hidden Ingredient In New, Greener Battery: A Virus. npr.org; National Public Radio. Zandonella, Catherine (11 April 2009). "Battery grown from "armour plated" viruses". New Scientist Bullis, Kevin (28 September 2006). "Powerful Batteries That Assemble Themselves". technologyreview "Bad Virus Put to Good Use". Clark School of Engineering, University of Maryland. 6 December 2010. "Self-Assembled Nanocomposites Boost Lithium-Ion Battery Anodes". Nature Materials http://www.gatech.edu/newsroom/release.html?nid=54920. "New Nanowire Battery Holds 10 Times The Charge Of Existing Ones". Science Daily. 20 December 2007. http://www.sciencedaily.com/releases/2007/12/071219103105.htm. "Interview with Dr. Cui, Inventor of Silicon Nanowire Lithium-ion Battery Breakthrough". GM-Volt. "Metal hydrides for lithium-ion batteries". Nature Materials 7 (11): 916–921. 2008NatMa...7..916O "Laboratory of the Prof. Gleb Yushin". Nano-tech.gatech.edu. 31 August 2011. "Deformations in Si−Li Anodes Electrochemical Alloying in Nano-Confined Space". American Chemical Society (30 September 2011). "A Simple Way to Boost Battery Storage". Technology Review Welcome to Ener1. Ener1 (Press release). Archived from the original 8 July 2006. EnerDel Technical Presentation (PDF). EnerDel Corporation. 29 October 2007. Bullis, Kevin (22 June 2006). Higher-Capacity Lithium-Ion Batteries Technology Review "How to Prolong Lithium-based Batteries". Battery University "Modelling Lithium-ion cells, Analysis Lithium-Ion Battery Degradation during Thermal Aging" (PDF). http://www.electrochem.org/dl/ma/204/pdfs/0253.PDF About Battery Management Systems White Paper - CCCV chargers: a false sense of security. ELithion LLC. Commercial Power (9 September 2006). "Safety handling guidelines for Lithium Batteries" (PDF). http://marine.rutgers.edu. University. "Safety Last". The New York Times. http://www.nytimes.com/2006/09/01/opinion/01cringely.html Nokia issues BL-5C battery warning, offers replacement. Wikinews. 14 August 2007. Staff (27 July 2007). Nokia N91 cell phone explodes Mukamo - Filipino News (blog). "Dell Recalls Lithium Batteries". Chemical and Engineering News:11; American Chemical Society Dell laptop explodes at Japanese conference. The Inquirer. "Kyocera Launches Precautionary Battery Recall" (Press release). Kyocera Wireless. 28 October 2004. "Safe Travel". Safetravel.dot.gov. U.S. Department of Transportation. 1 January 2008 "U.S. Department of Transportation revises lithium battery rules press release". Little Guy Media. http://www.robgalbraith.com/bins/content_page.asp?cid=7-9206-9211 Prohibitions - 6.3.12 - Dangerous, offensive and indecent articles (PDF). Hong Kong. December 2009 International Mail > FAQs > Goods/Services: Shipping a Laptop Japan Post Service Co. Ltd. http://arstechnica.com/science/news/2011/08/new-solid-state-compound-lithium-ion-batteries.ars Melody Voth (6 December 2010). "Battery Booster". http://pubs.acs.org/cen/email/html/8849bus1.html "What Are Batteries, Fuel Cells, and Supercapacitors?" (PDF). Chemical Review 104 (104): 4245. doi:10.1021/cr020730k. http://pubs.acs.org/doi/pdf/10.1021/cr020730k. Retrieved 25 July 2010. Lithium batteries at the Open Directory Project "The Future of Electric Vehicles on Lithium Availability". Journal of Energy Security
Automotive Development
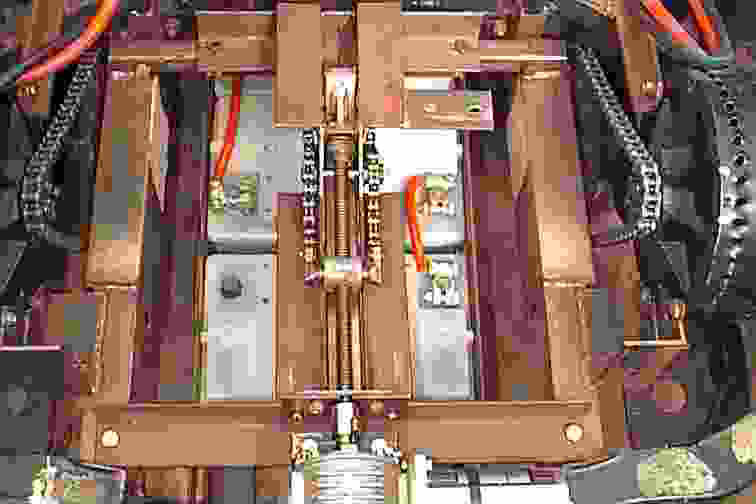
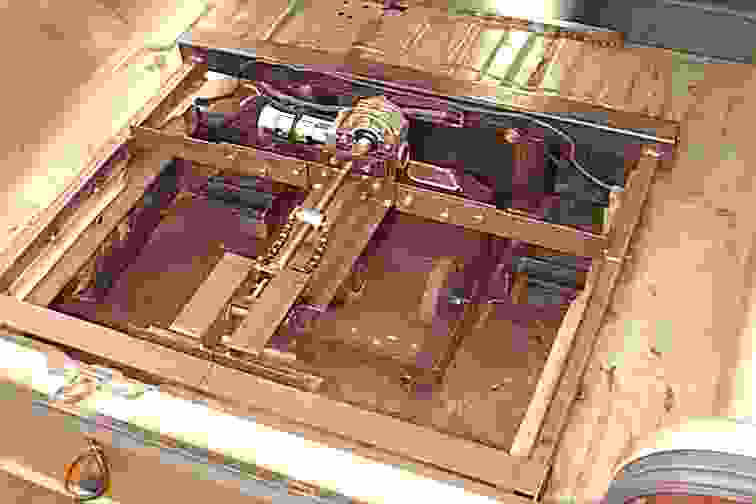
Battery cartridges are the way to go for road cars. The designer of Solarnavigator has already built several cars featuring battery cartridge exchange, as seen below. Lithium Ion batteries offer a much higher energy density and are ideal for extending the range between cartridge exchanges. See the pictures below for examples.
Intelligent Battery Support System
|
||||||||||||||||||||||||||||||||||||||
|
This website is copyright © 2013 Electrick Publications. All rights reserved. The bird logo and names Solar Navigator and Blueplanet Ecostar are trademarks ™. The Blueplanet vehicle configuration is registered ®. All other trademarks hereby acknowledged and please note that this project should not be confused with the Australian: 'World Solar Challenge'™which is a superb road vehicle endurance race from Darwin to Adelaide. Max Energy Limited is an educational charity working for world peace.
|